National Comprehensive Cancer Network® (NCCN®) Recommendations for JEMPERLI
You are about to leave a GSK website
You are about to leave a GSK website. By clicking this link, you will be taken to a website that is independent from GSK. The site you are linking to is not controlled or endorsed by GSK and GSK is not responsible for its content.
Trial results from RUBY Part 1
In All-Comers With Primary Advanced or Recurrent EC
At 3+ years, JEMPERLI + CP has the longest median follow-up for an FDA-approved immunotherapy combination to date1-6*
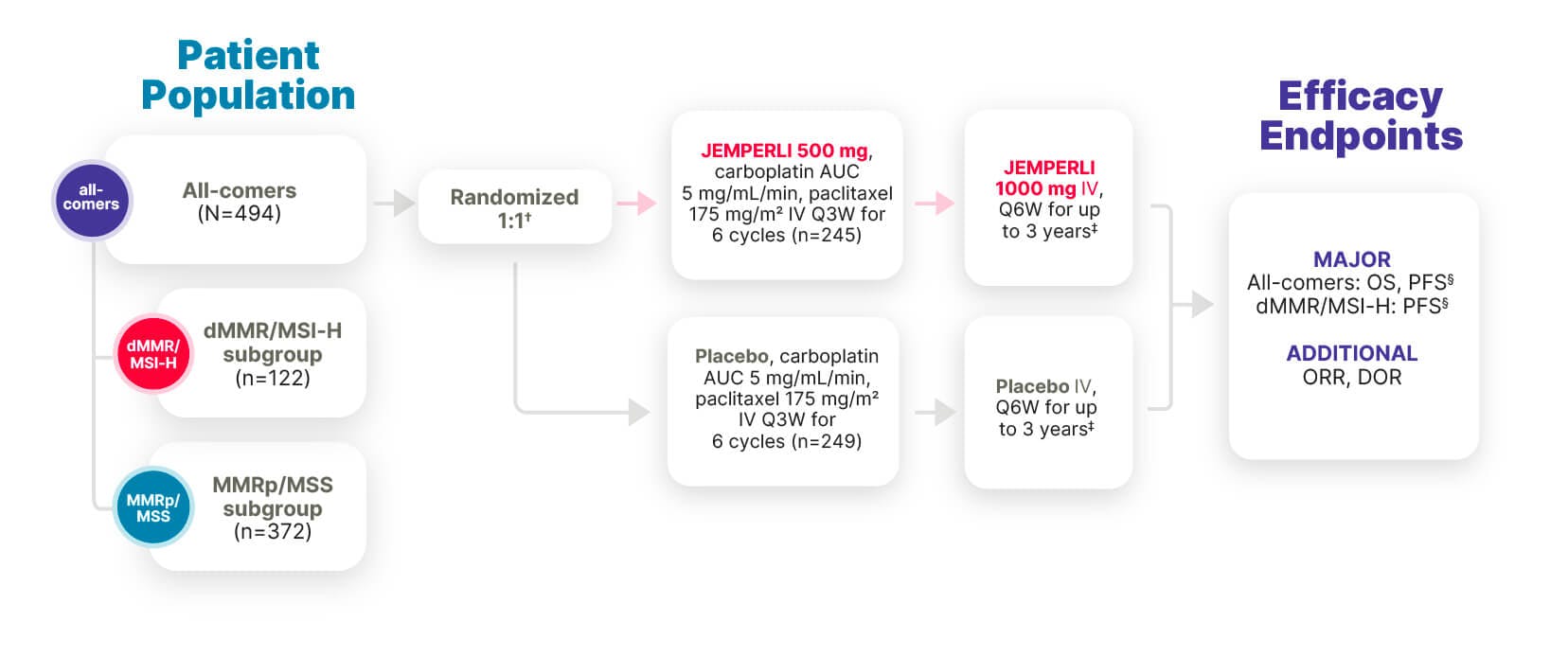
- All-comers is defined as the overall population with primary advanced or recurrent EC.
- * Median duration of follow-up, defined as time from randomization to data cutoff, was 37.2 months (cutoff date September 22, 2023).6
- † Randomization was stratified by MMR/MSI status, prior external pelvic radiotherapy, and disease status (recurrent, primary Stage III, or primary Stage IV).1
- ‡ Treatment continued until disease progression, unacceptable toxicity, or a maximum of 3 years.1
- § PFS assessed by the investigator according to RECIST v1.1.1
- AUC=area under the curve; CP=carboplatin-paclitaxel; dMMR=mismatch repair deficient; DOR=duration of response; EC=endometrial cancer;
IV=intravenous; MMR=mismatch repair; MMRp=mismatch repair proficient; MSI=microsatellite instability; MSI-H=microsatellite instability-high;
MSS=microsatellite stable; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; Q3W=every 3 weeks; Q6W=every 6 weeks; RECIST v1.1=Response Evaluation Criteria in Solid Tumors v1.1.
In Primary Advanced or Recurrent EC
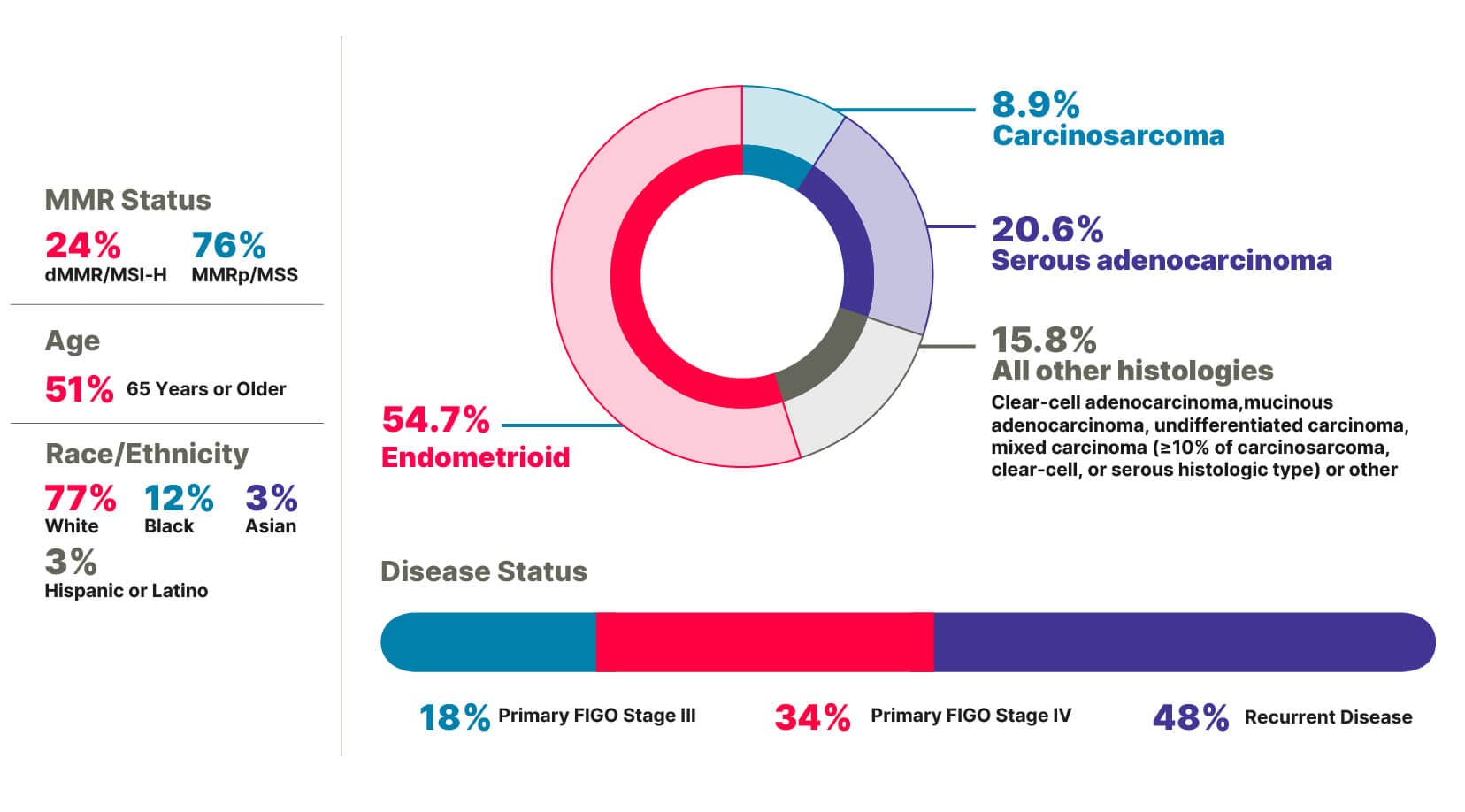
RUBY Part 1 included patients with broad disease characteristics1,7
Primary FIGO Stage III or Stage IV disease, including patients with more aggressive histologies such as carcinosarcoma and serous adenocarcinoma1,7-9
Measurable Disease1‖
Stage IIIA-IIIC1
Measurable‖ or Non-Measurable Disease1
Stage IIIC1 patients with carcinosarcoma, clear cell, serous, or mixed histology (≥10% carcinosarcoma, clear cell, or serous histology)
Stage IIIC2 or IV
First recurrent endometrial cancer with a low potential for cure by radiation therapy or surgery alone or in combination, including those1:
- Naïve to systemic anticancer therapy
- Who had received prior neoadjuvant/adjuvant systemic anticancer therapy and who had a recurrence or disease progression ≥6 months after completing treatment (first recurrence)
All patients were anti–PD-1/L1/L2 naïve10
‖ Measurable or evaluable by RECIST v1.1.1
FIGO=International Federation of Gynecology and Obstetrics; PD-1=programmed death receptor 1; PD-L1/2=programmed death receptor ligand 1/2.
RUBY Part 1 included patients with diverse disease characteristics (N=494)1,7
Major Endpoint
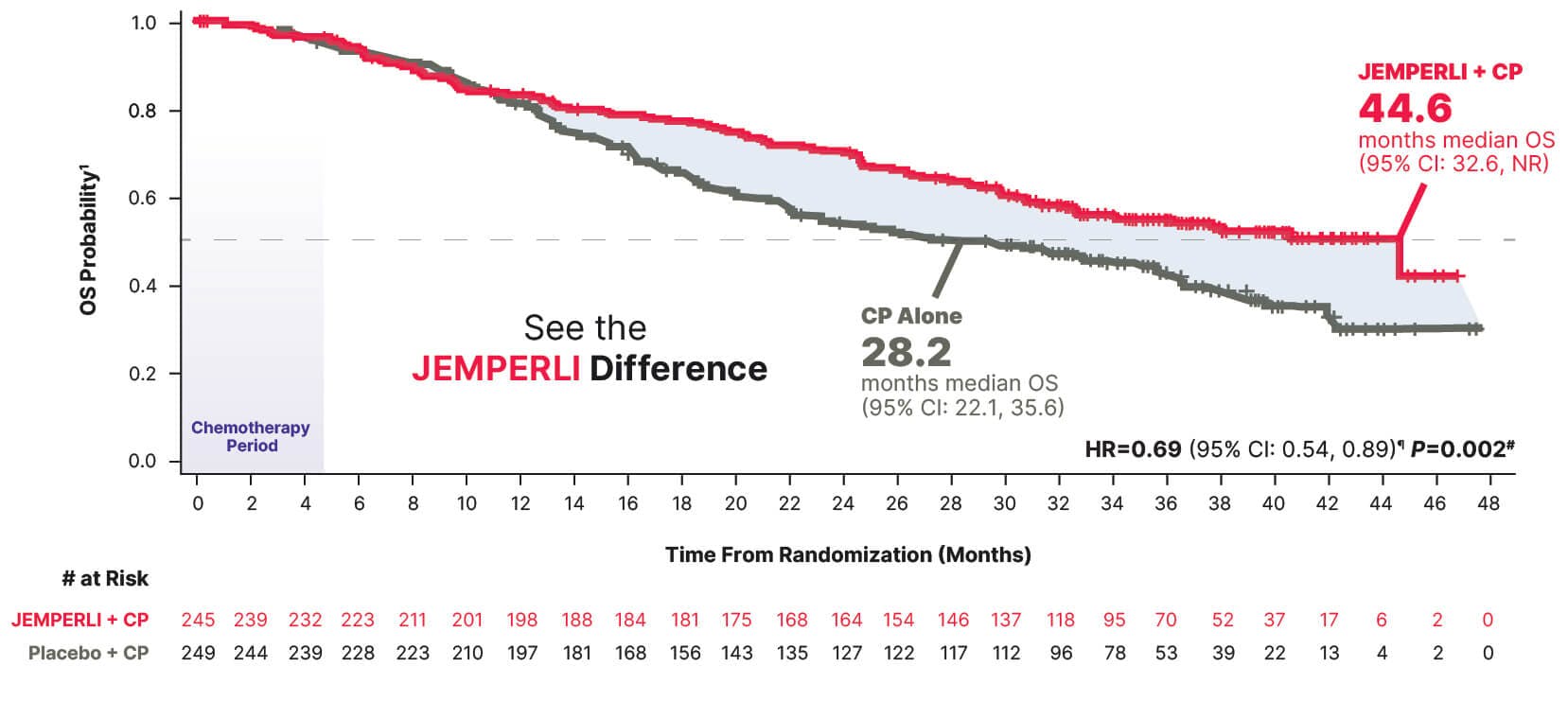
16-Month improvement in median overall survival vs CP alone1
Statistically significant 31% reduction in the risk of death with JEMPERLI + CP vs CP alone1

Estimated Kaplan-Meier probability of overall survival at 24 months was 70.1% (95% CI: 63.8, 75.5) with JEMPERLI + CP and 54.3% (95% CI: 47.8, 60.3) with CP alone6
Data cutoff September 22, 2023.6
¶ Based on stratified Cox regression model.1
# One-sided P-value based on stratified log-rank test was statistically significant.1
Major Endpoint
~1-Year median PFS in a population that included those with aggressive histologies1,7-9
Statistically significant 36% reduction in the risk of progression or death with JEMPERLI + CP vs CP alone1
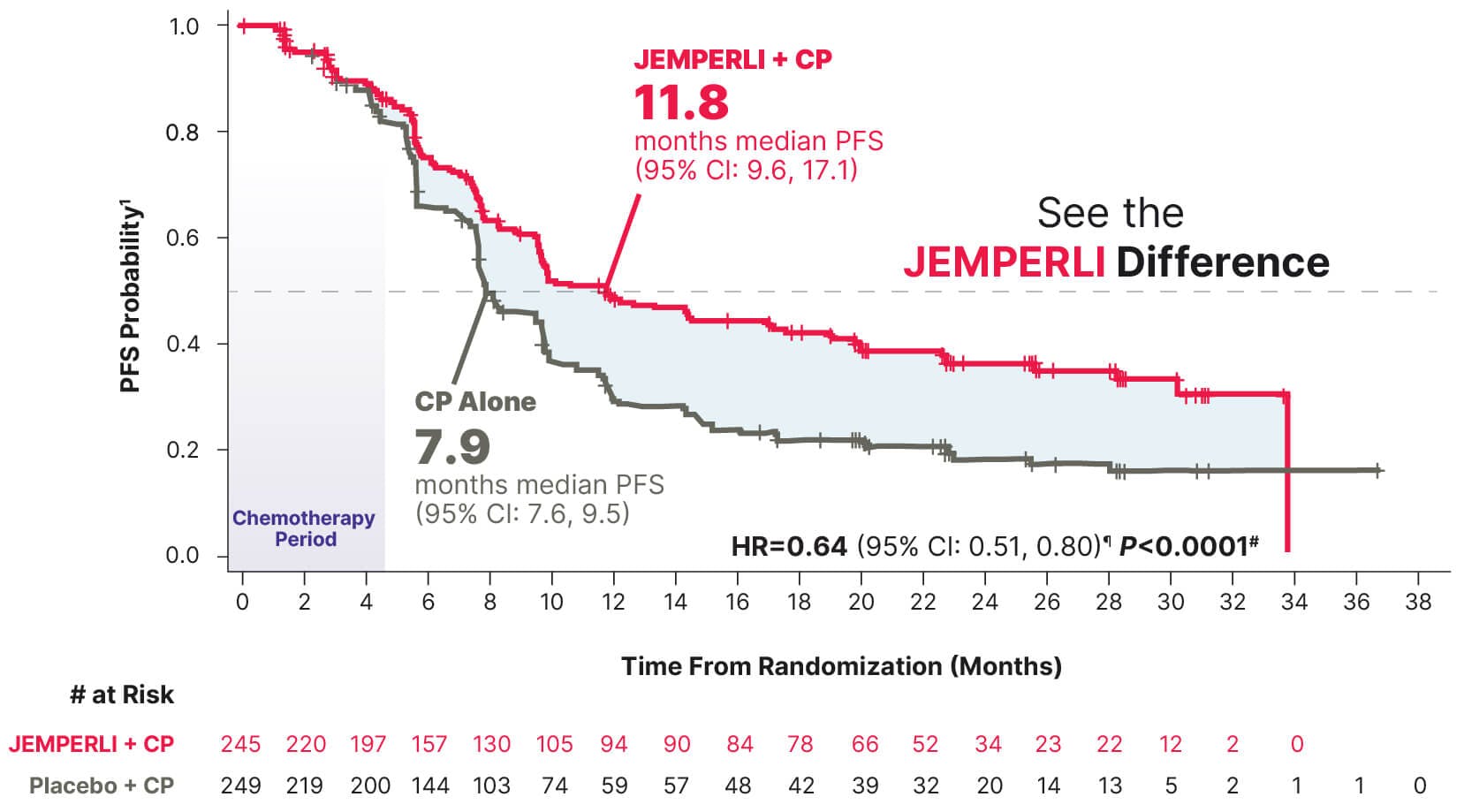
Data cutoff September 28, 2022.7
¶ Based on stratified Cox regression model.1
# One-sided P-value based on stratified log-rank test was statistically significant.1
Major Endpoint
~2-Year improvement (22.6 months) in median PFS vs CP alone1
Groundbreaking 71% reduction in the risk of progression or death with JEMPERLI + CP vs CP alone1
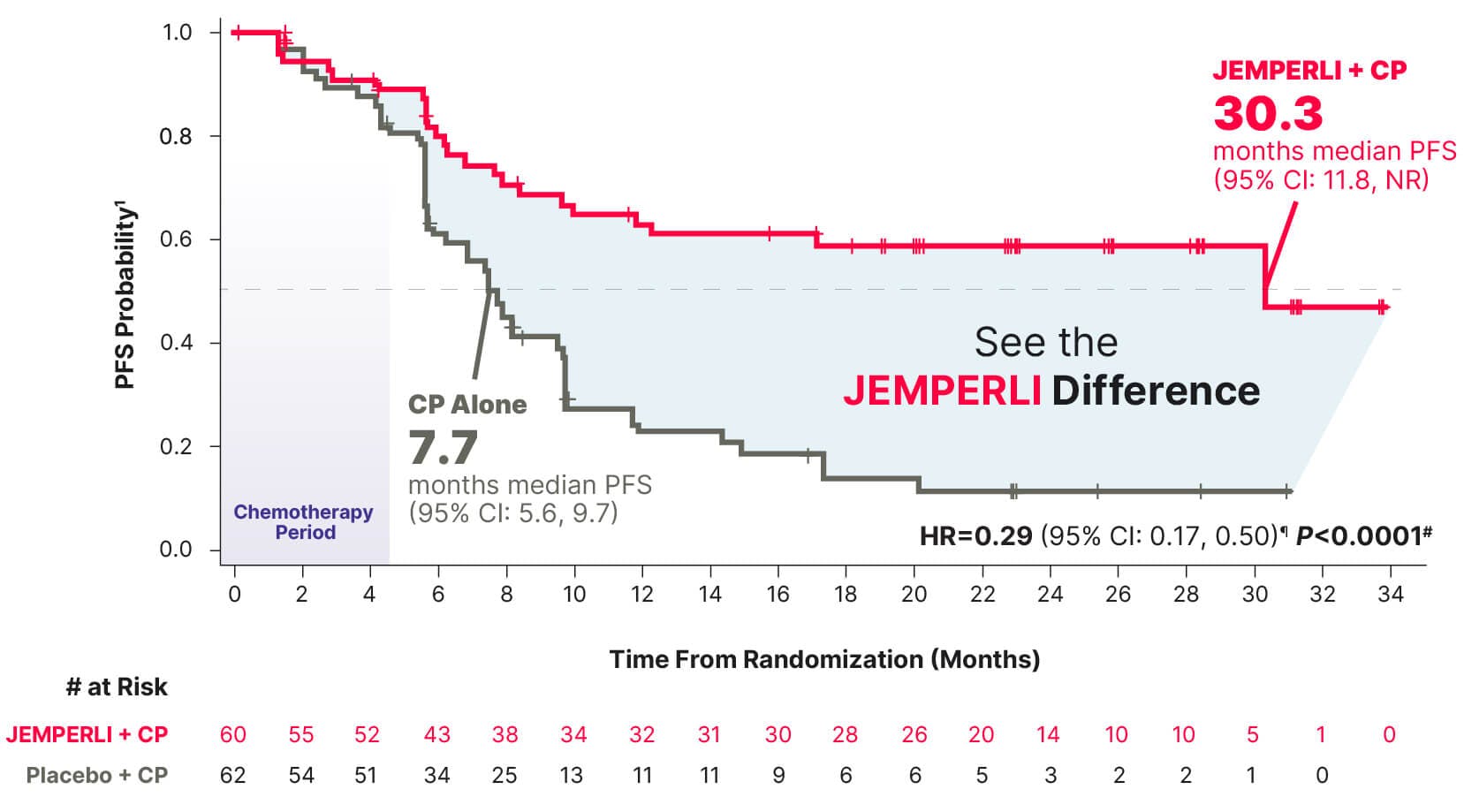
Data cutoff September 28, 2022.7
¶ Based on stratified Cox regression model.1
# One-sided P-value based on stratified log-rank test was statistically significant.1
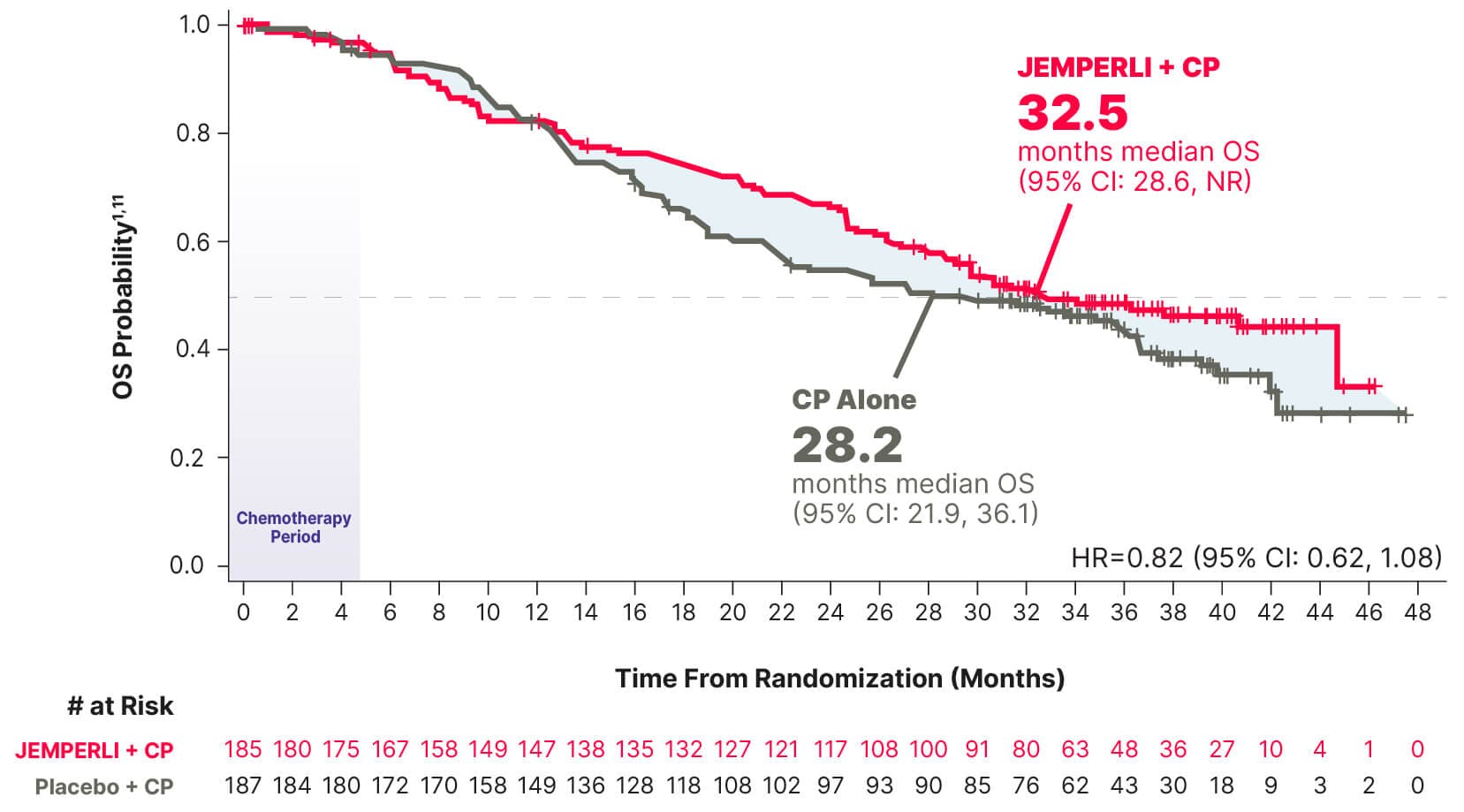
Exploratory Analysis
Median overall survival was not reached with JEMPERLI + CP and 30.8 months with CP alone1,11
The prespecified exploratory analysis for overall survival was not powered to detect treatment differences; results are descriptive1

Estimated Kaplan-Meier probability of overall survival at 24 months was 81.4% (95% CI: 68.9, 89.2) with JEMPERLI + CP and 53.6% (95% CI: 40.3, 65.3) with CP alone11
Data cutoff September 22, 2023.6
¶ Based on stratified Cox regression model.1
Exploratory Analyses
Improvement in overall survival observed with JEMPERLI + CP1,11
The prespecified exploratory analyses for overall survival and PFS were not powered to detect treatment differences; results are descriptive1
- 76% of patients in the overall population had MMRp/MSS biomarker status (n=372)1
PFS Analysis
Median PFS was 9.8 months (95% CI: 9.0, 12.6) for JEMPERLI + CP (n=185) vs 7.9 months (95% CI: 7.6, 9.8) for CP alone (n=187), and HR=0.78 (95% CI: 0.60, 1.00)1
OS data cutoff September 22, 2023.6
PFS data cutoff September 28, 2022.7
Safety profile from the RUBY Part 1 trial
RUBY Part 1 patient profiles
JEMPERLI dosing and administration
To report SUSPECTED ADVERSE REACTIONS, contact GSK at gsk.public.reportum.com or 1-888-825-5249 or
FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References
JEMPERLI. Prescribing information. GSK; 2025.
Eskander RN, et al. N Engl J Med. 2023;388(23):2159-2170.
Westin MR, et al. J Clin Oncol. 2023;42(3):283-299.
KEYTRUDA. Prescribing information. Merck & Co, Inc; 2025.
IMFINZI. Prescribing information. AstraZeneca Pharmaceuticals LP; 2025.
Powell MA, et al. Ann Oncol. 2024;35(8):728-738.
Mirza MR, et al. N Engl J Med. 2023;388(23):2145-2158.
Bogani G, et al. Int J Gynecol Cancer. 2023;33(2):147-174.
Clarke MA, et al. J Clin Oncol. 2019;37(22):1895-1908.
ClinicalTrials.gov. Accessed September 14, 2025. https://clinicaltrials.gov/study/NCT03981796
Data on file, GSK.

